How to Prepare for a Regulatory Audit
How to Prepare for a Regulatory Audit
Blog Article
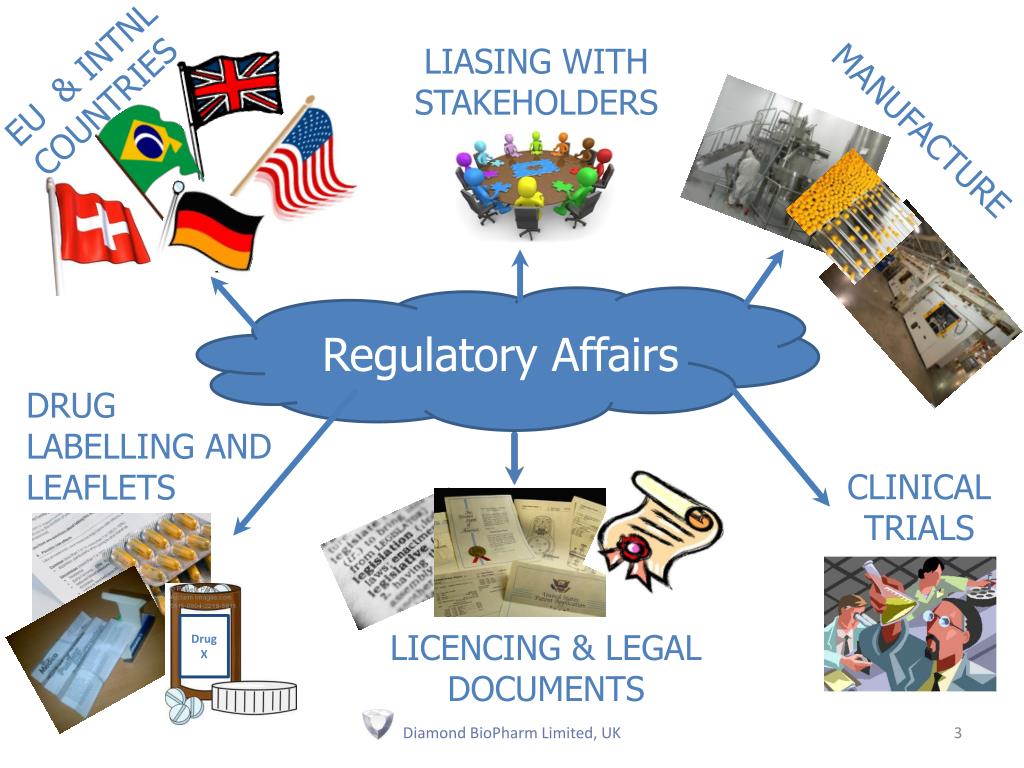
In today's rapidly evolving regulatory landscape, preparing for a regulatory audit is more crucial than ever. Regulatory affairs play a vital role in ensuring that companies comply with the various laws and regulations governing their industries. Whether you are in pharmaceuticals, biotechnology, medical devices, or any other regulated field, understanding how to effectively prepare for an audit can make all the difference between a smooth process and potential setbacks.
A regulatory audit can be a daunting experience, but with proper preparation, organizations can confidently showcase their compliance efforts. The audit process involves a thorough examination of records, practices, and systems to assess adherence to relevant regulations. By taking proactive steps to ensure readiness, companies can not only mitigate risks but also demonstrate their commitment to maintaining high standards of quality and safety.
Understanding Regulatory Requirements
In the realm of regulatory affairs, understanding the specific requirements mandated by governing bodies is crucial for successfully navigating audits. Each industry, whether pharmaceuticals, medical devices, or food safety, has its own set of regulations that must be adhered to. Familiarizing yourself with these regulations is the first step in ensuring compliance and avoiding potential pitfalls during an audit. Organizations must stay updated on any changes to regulations, as these can significantly impact operations and compliance strategies.
Establishing a robust compliance framework is essential for meeting regulatory requirements. This framework should incorporate policies and procedures that align with the regulatory standards relevant to your industry. It is also important to engage with stakeholders, including legal experts and compliance officers, to ensure that all aspects of the regulations are being interpreted and implemented correctly. Regular training and communication within the organization can foster a culture of compliance, making it easier to prepare for audits.
Documentation plays a vital role in demonstrating compliance with regulatory requirements. Maintaining detailed records of all compliance activities, from training sessions to quality control checks, provides evidence during an audit. A well-organized documentation system not only helps in fulfilling regulatory obligations but also streamlines the audit process. By ensuring that all necessary documentation is accurate, complete, and readily accessible, organizations can enhance their readiness for regulatory audits and minimize the risk of non-compliance findings.
Developing an Audit Preparation Plan
Creating a comprehensive audit preparation plan is essential for successfully navigating a regulatory audit. Start by assessing the specific regulations and standards applicable to your organization. This involves gathering relevant documents, understanding compliance requirements, and identifying key stakeholders who will play a role in the audit process. By mapping out these components, you can clarify what needs to be documented and prepared, thereby streamlining the subsequent steps.
Next, establish a timeline that outlines the milestones leading up to the audit. This timeline should include deadlines for completing necessary documentation, training staff on compliance procedures, and conducting internal reviews. Regular checkpoints will help ensure that everyone remains aligned and that any potential issues can be addressed well in advance. Engage your team members by assigning clear responsibilities, which fosters a collaborative environment and encourages accountability.
Finally, conduct mock audits as a rehearsal for the actual regulatory audit. This practice will help identify gaps in your compliance processes and give your team an opportunity to experience the audit environment. Use feedback from these mock sessions to make necessary adjustments and improvements to your preparation efforts. Ultimately, a well-structured audit preparation plan positions your organization to demonstrate its commitment to regulatory compliance and enhances the likelihood of a successful audit outcome.
Conducting a Mock Audit
Regulatory Affairs Program
Conducting a mock audit is a crucial step in preparing for a regulatory audit. This proactive approach allows organizations to simulate the regulatory audit environment, helping to identify gaps in compliance and areas needing improvement. By running through the audit processes beforehand, teams can gain insights into how well their processes align with regulatory requirements and what adjustments may be necessary to ensure compliance.
During the mock audit, all relevant documentation, procedures, and employee roles should be reviewed thoroughly. It is essential to analyze how effectively each department adheres to established regulatory standards. Engaging various stakeholders, including regulatory affairs personnel, quality assurance teams, and department heads, can provide a comprehensive overview of the organization’s preparedness and responsiveness to potential audit challenges.
After the mock audit concludes, a detailed analysis of the findings should be conducted. This evaluation should lead to an action plan that addresses identified issues while promoting continuous improvement. Regular mock audits can foster a culture of compliance within the organization, ensuring that regulatory affairs are consistently upheld and enhancing overall readiness for actual audits.
Report this page