Regulatory Affairs 101: The Basics You Need to Know
Regulatory Affairs 101: The Basics You Need to Know
Blog Article
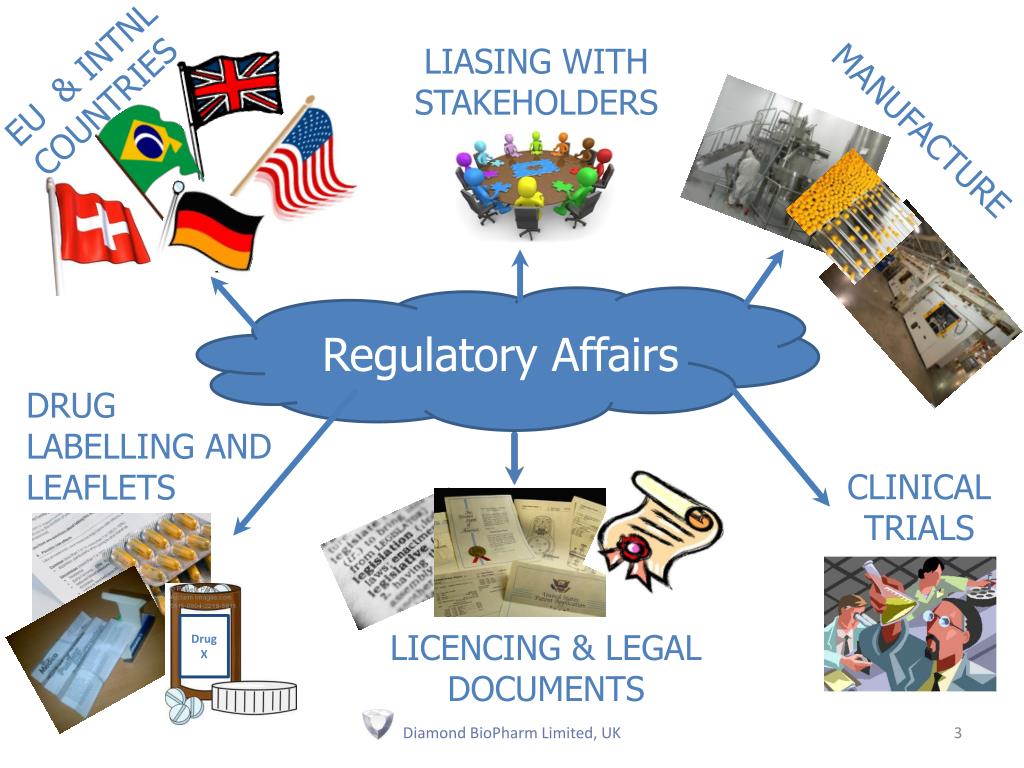
Regulatory Affairs is a crucial field that plays a significant role in ensuring that products, particularly in the healthcare and pharmaceuticals sectors, meet all necessary standards for safety and efficacy. As industries become increasingly complex and globalized, the importance of skilled regulatory professionals has only grown. Their work helps to navigate the intricate web of regulations, guidelines, and requirements imposed by governments and regulatory bodies.
For those new to the field, understanding the basics of Regulatory Affairs can seem daunting. However, gaining a solid foundation can empower individuals and organizations to successfully bring innovative products to market while ensuring compliance with legal and ethical standards. This article aims to demystify the essentials of Regulatory Affairs and provide insights that are valuable for both newcomers and seasoned professionals alike.
Key Roles in Regulatory Affairs
Regulatory Affairs professionals play a vital role in ensuring that products meet necessary legal standards before they reach the market. They are responsible for the strategic planning and execution of all regulatory activities, including the submission of documents to government agencies. This involves a deep understanding of various regulations and guidelines that govern the industry, whether it be pharmaceuticals, biotechnology, or medical devices. Their expertise allows companies to navigate complex regulatory landscapes efficiently.
Another key role within Regulatory Affairs is that of the Regulatory Affairs Specialist. These individuals focus on gathering and analyzing data related to product compliance and safety. They prepare and review technical documentation required for regulatory submissions, ensuring that all information is accurate and meets the standards set forth by regulatory authorities. Their attention to detail is crucial in preventing delays in product approval and ensuring a smooth pathway to market.
Finally, the Regulatory Affairs Manager oversees the entire regulatory process and team, coordinating efforts between departments such as research and development, quality assurance, and marketing. This role often requires strategic leadership and effective communication skills to align regulatory goals with business objectives. Regulatory Affairs Managers also work closely with external stakeholders, including regulatory bodies and industry groups, to advocate for the company's interests and stay informed about emerging regulations that may impact future developments.
Understanding Regulatory Frameworks
Regulatory affairs operate within a complex landscape of legal and ethical standards that govern the development, approval, and marketing of products. This framework ensures that organizations comply with regulations set by government authorities, which vary significantly across different regions. Understanding these regulations is crucial for companies involved in pharmaceuticals, medical devices, and other industries that require rigorous oversight to protect public health and safety.
At the core of regulatory frameworks are the key agencies such as the Food and Drug Administration in the United States and the European Medicines Agency in Europe. These organizations establish guidelines that define the processes for clinical trials, product submissions, and post-market surveillance. Navigating these regulations requires a solid comprehension of the laws and policies that dictate how products must be tested and approved before reaching the market.
Regulatory Affairs Training
Moreover, regulatory frameworks are continuously evolving due to advancements in technology and changes in consumer needs. As new therapies and innovations emerge, regulatory bodies adapt their policies to ensure that safety and efficacy are always prioritized. Professionals in regulatory affairs must stay informed about these changes and engage in proactive communication with regulatory agencies to effectively manage compliance and mitigate risks associated with non-compliance.
Common Challenges in Regulatory Affairs
One of the primary challenges in regulatory affairs is keeping up with the constantly evolving regulations. Regulatory environments vary by country and region, making it difficult for professionals to stay informed about the latest changes and requirements. This necessitates a continuous learning approach, where regulatory affairs professionals must engage in ongoing education and networking to ensure compliance with local and international rules.
Another significant challenge is the complexity of product submissions and approvals. Regulatory submissions often require a comprehensive understanding of scientific data, clinical trials, and manufacturing processes. Navigating through these intricate details can be daunting, especially when different regulatory bodies have varying expectations regarding documentation and evidence. This complexity can lead to delays in product launches if not managed effectively.
Finally, communication between various stakeholders can pose difficulties in regulatory affairs. Collaboration among teams such as research and development, legal, and marketing is crucial to ensure that everyone is aligned with regulatory requirements. Miscommunication or lack of coordination can result in errors or omissions in submissions, jeopardizing approval timelines and contributing to increased costs. Balancing the needs of different departments while maintaining compliance is an ongoing challenge within the field.
Report this page